媒體教育 Trial updates: The role of early docetaxel with ADT in metastatic hormone-sensitive prostate cancer
Dr Lee Siu Hong
Specialist in Clinical Oncology
Introduction
Traditionally, metastatic hormone-sensitive prostate cancer is treated with androgen deprivation therapy (ADT), with a median overall survival (OS) of approximately 49 months. Despite good response to ADT, most patients will eventually develop castration- resistant cancer.
Docetaxel is an active agent in metastatic, castration-resistant prostate cancer. It significantly improved OS as reported in two phase III trials. The established efficacy of docetaxel in castration-resistant disease led to the evaluation of its use earlier in the disease course. It was postulated that upfront chemotherapy may improve the therapeutic benefit from ADT in hormone-sensitive cancer before the onset of resistance.
Two recently reported randomized trials evaluated the role of early docetaxel in hormone-naïve patients with metastatic or high-risk prostate cancer. Their findings offer a strategy to prolong survival in a select population of men with prostate cancer, and have the potential to transform current practice.
Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) trial were reported during the 2014 annual meeting of the American Society of Clinical Oncology (ASCO 2014). CHAARTED enrolled 790 men with hormone-naïve metastatic prostate cancer and randomized them to receive either ADT alone or ADT plus 75 mg/m2 docetaxel (ADT+D) every 3 weeks, for a maximum of six cycles. Upon enrollment, patients were stratified based on extent of metastatic disease into “high-volume” and “low-volume” groups. Those with high-volume disease had visceral metastases and/or four or more bone metastases (with at least one beyond the pelvis and vertebral column).
Over a median follow-up of 29 months, 136 deaths were reported in the ADT group and 101 deaths in the ADT+D group. The addition of docetaxel of ADT significantly improved the primary endpoint of OS, with a median of 57.6 months versus 44.0 months with ADT alone (hazard ratio [HR] 0.61; 95% CONFIDENCE INTERVAL [Cl], 0.47-0.80; p=0.0003). Specifically, OS in patients with high-volume disease significantly improved from 32.2 months to 49.2 months (HR 0.60; 95% Cl 0.45-0.81; p=0.0006) Adding docetaxel to ADT also produced higher prostate-specific antigen (PSA) responses, longer median time to castration resistance and longer median time to clinical progression compared with ADT alone (all, p<t;0.0001).
E3805 CHAARTED: Early docetaxel with ADT improves survival
Results of the E3805 Chemo-Hormonal Therapy
STAMPEDE backs CHAARTED findings
The Systemic Therapy in Advancing or Metastatic
Figure. Patients with detectable metastasis: ADT with docetaxel significantly improved OS 8
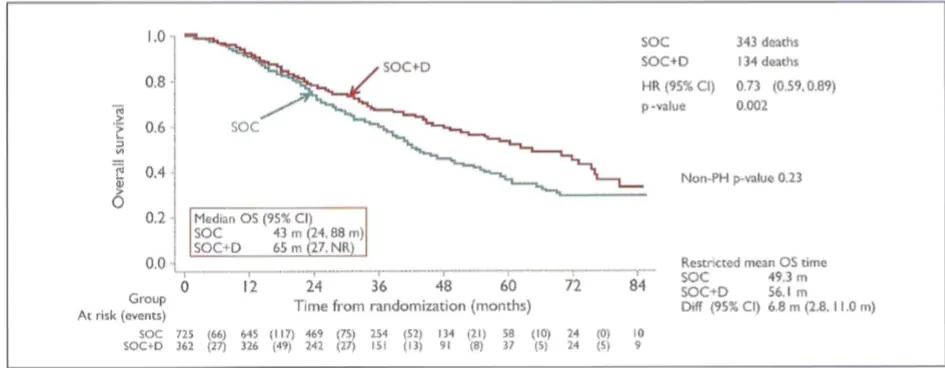
SOC, standard of care; D, docetaxel; ADT, androgen deprivation therapy; OS, overall survival; HR, hazard ratio; Cl, confidence; NR, not reached; m, months; PH, proportional hazards.
This newsletter is distributed as an independent supplement to MIMS Oncology.
Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) trial is a phase III, multi-arm study that was designed to assess the addition of specific treatment strategies to standard of care (SOC). To date, it is the largest randomized trial of prostate cancer treatment with almost 7,000 men recruited since 2005.
The study included men with high-risk, hormone-naïve, locally advanced or metastatic prostate cancer. The preliminary survival results were recently recently presented at ASCO 2015. In the present analysis, 2,962 men were assigned to four groups: SOC (control) (ADT ≥3 years plus radiation for suitable patients); SOC plus docetaxel (SOC+D); SOC plus zoledronic acid (SOC+ZA); and SOC plus both docetaxel
and zoledronic acid (SOC+D+ZA). Docetaxel was given at 75 mg/m2 every 3 weeks for 6 cycles, with prednisolone 10 mg daily. Zoledronic acid 4 mg was given every 3 weeks for 6 cycles, then every 4 weeks until 2 years. The primary endpoint was OS.
The study had a median follow-up of 42 months, with 405 reported deaths in the control arm. The cause of death in 84% of deaths was prostate cancer.
The addition of docetaxel to SOC significantly improved survival by 10 months (77 months, SOC+D versus 67 months, SOC; HR 0.76; 95% Cl 0.63-0.91; p=0.003) In contrast, survival was not significantly improved with SOC+ZA (versus SOC; HR 0.93; 95% Cl 0.79-1.11; p=0.437).
Notably, among patients with detectable metastasis (61% of total), survival significantly Table. Grade 3+ adverse events ever reported 8

SOC, standard of care; D, docetaxel; ZA, zoledronic acid; AE, adverse event; G5, grade 5.
Improved by 22 months with the addition of docetaxel (65 months, S0C+D versus 43 months, SOC; HR 0.73; 95% Cl 0.59-0.89; p=0.002) (Figure).
With regard to toxicity, 51%of men in the SOC+D group reported grade 3-5 toxicities compared with 31%of men in the SOC group (Table). The additional adverse effects were manageable and led to few discontinuations.
Summary and conclusion
The observed survival benefit from the addition of docetaxel to ADT was:
-
For all patients – 10 months in STAMPEDE; 13.6 months in CHAARTED;
-
For patients with metastasis, 22 months (STAMPEDE); for patients with high-volume disease, 17 months (CHAARTED).
At present, findings from both studies strongly suggest that patients with hormone-naïve, metastatic prostate cancer, particularly patients with high-volume disease, or high-risk, progressive prostate cancer, who can tolerate chemotherapy, may benefit from the upfront addition of six cycles of docetaxel to ADT. Clinicians should consider early chemotherapy with ADT as initial treatment for suitable patients due to the conferred survival advantage.